Abstract
Background: Venetoclax (VEN), an oral B-cell lymphoma 2 inhibitor, is approved for use in adult patients (pts) with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). As a targeted and highly active antitumor agent, VEN induces rapid and profound tumor reduction. Inpatient monitoring for initial doses of VEN is recommended by US Prescribing Information for pts with medium tumor burden and reduced renal function or high tumor burden. Administration of debulking agents, such as obinutuzumab (G), help reduce tumor burden and, consequently, facilitate subsequent administration of VEN in the outpatient setting. However, tumor reduction data are needed to definitively establish the utility of a debulking strategy. This study performed disease restaging after every 2 cycles of debulking to evaluate the safety and efficacy of G ± bendamustine (B) as a debulking regimen before VEN treatment in the outpatient community setting. The safety and efficacy of subsequent VEN+G treatment after debulking was also evaluated.
Methods: This open-label, Phase 3b study (NCT03406156) enrolled adult pts with previously untreated CLL/SLL (except those with 17p deletion) who had medium (any lymph node [LN] 5 to <10 cm or absolute lymphocyte count [ALC] ≥25×10 9/L) or high (any LN ≥10 cm or any LN ≥5 cm and ALC ≥25×10 9/L) tumor burden. A maximum of six 28-day cycles of G±B were administered, and disease restaging was performed after every 2 cycles. Once low tumor burden was achieved (all LN <5 cm and ALC <25x10 9/L), VEN+G was administered for 5 cycles followed by VEN monotherapy for a total time on VEN of up to 1 year. Disease assessments were performed at the end of combination therapy (EoCT; 5 mo after last dose of G) and at the end of therapy (EoT; 3 mo after last dose of VEN), and peripheral blood was collected for assessment of minimal residual disease (MRD) using the clonoSEQ assay (Adaptive Biotechnologies). Undetectable MRD was defined as <1 CLL cell/10 4 leukocytes (<10 -4; uMRD4), <10 -5 (uMRD5), or <10 -6 (uMRD6). The primary endpoints were the percentage of pts achieving low tumor burden after 2, 4, and 6 cycles of G±B debulking and complete remission (CR) and CR with incomplete marrow recovery (CRi) rates among pts receiving VEN.
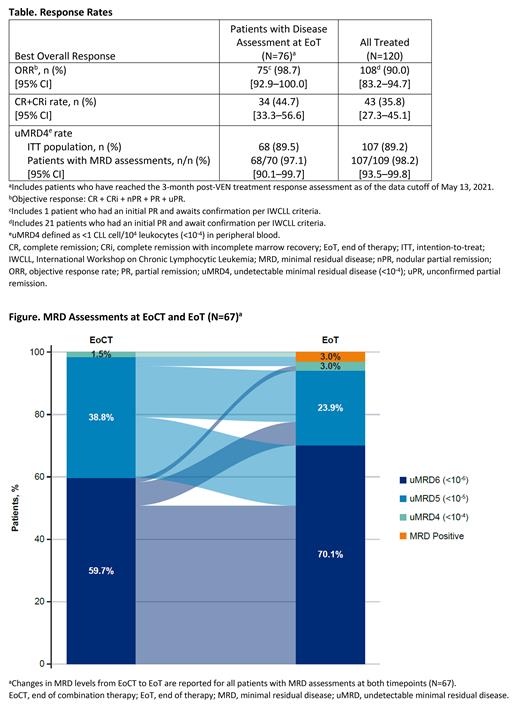
Results: Of 120 pts treated, 81 received G for debulking and 39 received G+B. As of 13 May 2021, 2 pts remained on study treatment, 108 were in posttreatment follow-up, and 10 had discontinued the study for reasons including death (n=7), withdrawn consent (n=2), and COVID-19 infection (n=1). At baseline, 82.5% of pts had ALC ≥25x10 9/L, 33.3% had LN ≥5 cm, and 24.2%/75.0%/0.8% had high/medium/low tumor burden, respectively. Low tumor burden was achieved in 91.6% (109/119) of evaluable pts receiving G±B debulking. In the all-treated population (N=120), the objective response rate (ORR) was 90.0% and the CR/CRi rate was 35.8%. Among pts receiving VEN with disease assessment at EoT (N=76), the ORR was 98.7% and the CR/CRi rate was 44.7% (Table). The best uMRD4 rates in peripheral blood were 89.2% (107/120) for all-treated and 98.2% (107/109) for evaluable pts. Among evaluable pts, the uMRD4 rates were 100% (100/100) and 97.1% (68/70) at EoCT and EoT, respectively. Among pts with MRD assessments at both timepoints (N=67), 19.4% had a deepening of their MRD response from EoCT to EoT, and 67.2% maintained the same MRD level (Figure). At a median follow-up of 24.0 mo, 7 deaths (6 related to COVID-19 infection and 1 from cardiac complication after pancreatic mass resection) and no incidences of disease progression were reported; the estimated 18-mo PFS was 94.1%. In pts treated with G vs G+B debulking, respectively, the incidences of Grade ≥3 TEAEs were 71.6% vs 84.6% (most common was neutropenia at 28.4% vs 41.0%) and serious AEs were 23.5% vs 17.9% (most common were pneumonia and COVID-19 pneumonia, each at 3.7% vs 2.6%).
Conclusion: In this study, most (91.6%) pts achieved low tumor burden after debulking. The uMRD4 rate was 98.2% among MRD-evaluable pts (89.2% among all pts), with 100% and 97.1% uMRD4 rates at EoCT and EoT, respectively. Overall, these results highlight the utility of G±B as an effective debulking strategy that can facilitate VEN treatment initiation in the outpatient setting. The efficacy and safety results are consistent with other VEN+G trials. Preventive measures for COVID-19 should be continuously emphasized for pts with CLL.
Flinn: AstraZeneca: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Merck: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Karyopharm Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Teva: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Janssen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Kite, a Gilead Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Genentech: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Trillium Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; BeiGene: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Novartis: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Loxo: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; ArQule: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Celgene: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Roche: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Constellation Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; AbbVie: Consultancy, Other: All Consultancy and Research Funding payments made to Sarah Cannon Research Institute, Research Funding; Portola Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Rhizen Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Incyte: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Infinity Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; IGM Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Forty Seven: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Forma Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Curis: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Verastem: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Seagen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Juno Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Gilead Sciences: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Acerta Pharma: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Agios: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Calithera Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Takeda: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Pfizer: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Iksuda Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Unum Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; TG Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; MorphoSys: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Nurix Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Great Point Partners: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Triphase Research & Development Corp.: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Century Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Hutchison MediPharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Vincerx Pharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Sarah Cannon Research Institute: Current Employment; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Seagen: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Unum Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute, Research Funding; Johnson & Johnson: Current holder of individual stocks in a privately-held company; Seattle Genetics: Research Funding. Andorsky: AbbVie: Research Funding; Celgene/Bristol Myers Squibb: Consultancy; Celgene/Bristol Myers Squibb: Research Funding; Epizyme: Research Funding; AstraZeneca: Other: served on steering committees; AbbVie: Consultancy. Melear: TG Therapeutics: Speakers Bureau; Astrazeneca: Speakers Bureau; Janssen: Speakers Bureau. Manda: Morphosys: Honoraria; Genmab: Current equity holder in publicly-traded company. Kolibaba: TG Therapeutics: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Atara Biotechm: Consultancy; McKesson Specialty Health: Consultancy; Sunitomo Dainippon Pharma: Consultancy; Tolero Pharma: Consultancy, Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Yimer: GSK: Speakers Bureau; Beigene: Speakers Bureau; Janssen: Speakers Bureau; Astrazeneca: Speakers Bureau; Karyopharm: Current equity holder in publicly-traded company, Speakers Bureau; Sanofi: Speakers Bureau; Amgen: Speakers Bureau; Pharmacyclics: Speakers Bureau; Texas Oncology: Current Employment. Burke: Kura: Consultancy; Epizyme: Consultancy; Kymera: Consultancy; Adaptive Biotechnologies: Consultancy; Roche/Genentech: Consultancy; Beigene: Consultancy, Speakers Bureau; MorphoSys: Consultancy; Verastem: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; Bristol Myers Squibb: Consultancy; X4 Pharmaceuticals: Consultancy; SeaGen: Consultancy, Speakers Bureau. Fanning: BMS: Speakers Bureau; TG Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genmab: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sanofi: Speakers Bureau; Takeda: Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees. Islas-Ohlmayer: OHC/USON: Current Employment; AbbVie: Honoraria; Rigel: Honoraria, Speakers Bureau. Vizkelety: AbbVie: Current Employment, Current equity holder in publicly-traded company. Pesko: AbbVie: Current Employment, Current equity holder in publicly-traded company. Chyla: AbbVie: Current Employment, Current equity holder in publicly-traded company. Jiang: AbbVie: Current Employment, Current equity holder in publicly-traded company. Sharman: Pharmacyclics LLC, an AbbVie Company: Consultancy; BMS: Consultancy; Lilly: Consultancy; BeiGene: Consultancy; Centessa: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy; TG Therapeutics: Consultancy; AbbVie: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal